|
4.2 Virus Vaccine
4.2.1 Flow sheet and Environment Distribution Map of Virus Vaccine
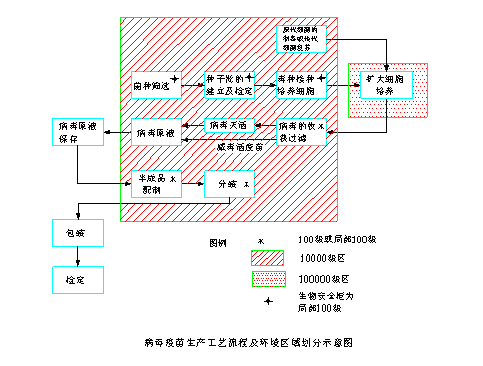
4.2.2 Key of Production Control
4.2.2.1 Material and adjuvant
The quality of materials should comply with updated " China Medicine Dictionary" or "China Bio-products Material Quality Stander ". Any chemical reagents, which are not included in the above standards, should be at least chemical purity.
£¨1£© All the material providers and their products should be inspected before signing a providing agreement.
Strict methods of materials management should be established and performed seriously.
4.2.2.2 Manufacture Tools
Any manufacture tools, which contact the products directly, should be cleaned and sterilized strictly before use.
Animal for manufacture and testing
Small mice, chicken embryos and other animals, which are used for manufacture, should be according to clean-class standard.
4.2.2.4
Bacteria seed for production should be within certain generations, with obvious bio-character, and non-contamination.
4.2.2.5
Virus seed for production should be without any outer contamination, identified completely for bio-feature, and stored in -20¡æ for freeze-dry virus, -60¡æ for liquid virus.
4.2.2.6
Virus seed's inoculation and culture should be performed in special equipment. Some of varieties must be in the state-approved lab P3, P2.
4.2.2.7
Culture-medium for production should be suitable for the growth of cell. During the production, any penicillium and other¦Â-lactam anti-microbial should not be added in.
4.2.2.8
Collected cell-created liquid from different bottles in same batch could be merged and sampled for identification.
4.2.2.9 During production of inactive vaccine, proper sterilizing agent should be added in for inactivation. Areas for active vaccine and inactive vaccine must be divided strictly.
4.2.2.10 Concentration ratio of sterilizing agent and little cattle serum should be limited strictly in vaccine products.
4.2.3 Key of Quality Control
Following processes are divided according to vaccine-producing technique.
Process A: Bacteria seeds and testing serums should be approved by CFDA and issued by CFDA-confirming agents.
Process B 1. The history, source and bio-character of original seed batch should be recorded. 2. Establish the main seed batch from original seed batch, the productive batch from main batch, and make all the batches have coincident character. 3. Identify the seed batch by following testing: culture character, serum character, poison-power, immune power, virus viscosity, outer gene and purity.
Process C: Virus identification: cell aberrance, viscosity and other special test.
Process D: Cell source, history confirm, cell figure observation, cell identification and outer source gene test.
Process E: Cell figure observation, cell identification and outer source gene test.
Process F: Virus purity test, virus viscosity test, and aseptic test. |