4.4 Cell Culture Medium
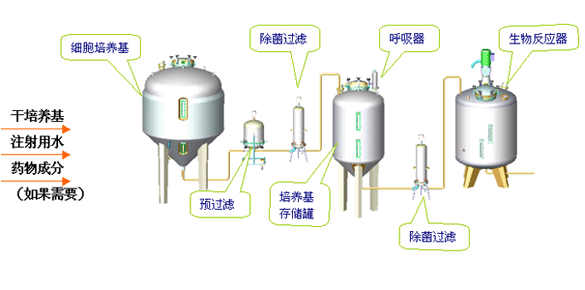
Production of medical protein cells requires the medium with exact nutrition ratio to ensure cell¡¯s multiplying and growth. All the nutrition substances are provided by culture medium, so the medium should be aseptic. If the culture medium contains any animal tissue components, the final filter should be capable of removing the mycoplasma.
Normally, culture mediums are prepared in mass quantity and stored in vessels. Before enter the fermentor or bio-reactor, filtered by bacteria-removing filtering system. Pre-filter is for particle and colloid removing, to extend the life of final filter.
Aim of Separation
Pre-filtering
Before final filtering, remove lipoids, colloids and particles.
Final-filtering
Remove bacteria and mycoplasmas
Requirement of Application
Pre-filtration should be effectively removing the particles and colloids, but not interfere the pass of important ingredients of culture-medium.
For economical and efficient separation, pre-filtration should be with continual high flow-rate. In addition, for the different batch may have different ingredient. The designed filtering system should be capable of acceptance the difference.
Recommended Filters
For culture-medium without serum, the pre-filter is not necessary. The final bacteria-removing filters should be Polyvinylidene fluonde PVDF 0.22¦Ìm
For serum-containing medium, the normal pre-filters¡¯ membranes are mixed-cellulose-ester or glass-cellulose. Both can provide high flow-rate and high retention-rate. For removing mycoplasma, Polyvinylidene fluonde 0.1¦Ìm membrane should be used. For air-filtration, poly-propylene is normal option for bacteria and virus removing.
|