|
4.8Plasma Products
4.8.1 Flow sheet and environment-dividing map
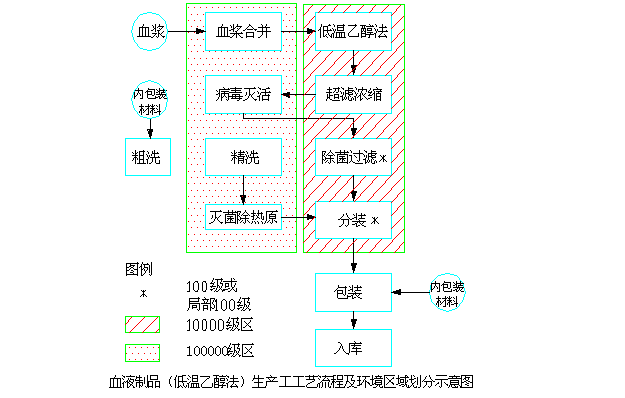
4.8.2 Key of Production Control
(1) Manufacture tools: Any metal or glass tools used for manufacture directly should be specialized and cleaned, sterilized, pyrogen-removed strictly before use.
(2) The collection and quality of material plasma should be according to the " regulation for material plasma collection". The plasma should be as aseptic as possible and stored under -20¡æ within 8 hours. Qualified plasma should be put into production or stored under -20¡æ. Storage period should not over 2 years.
(3) For protein's osmosis-separation and concentration, super filtration should be adopted for clarification and bacteria-removing. The medium should be non-asbestos.
(4) Each batch product should be heated to 60¡æ¡À0.5¡æcontinually for at least 10h to inactivate the remaining virus.
(5)Repacking and freeze-dry should be performed in absolute aseptic condition.
A¡¢After repacking, store in 20¡«25¡æ for 4 weeks or 30¡«32¡æ for 14 days, and inspect whether each bottle's appearance is qualified.
B¡¢Freeze-dry products should be frozen after repacking. The proper condition should be adopted for freeze-dry. The temperature during freeze-dry should not be over 50¡æ. The products should be vacuum-packed.
|